Overcoming the Biopharma Skills Gap
Compliance with evolving GMP requirements poses a significant challenge for today’s biopharma industry, and employee training has been identified as a key challenge for compliance and manufacturing success

Arnaud Schmutz
Sourcin CEO

Alain Pralong
Pharma-Consulting

Hélène Pora
Pall Corporate
The underlying reason for regulatory observations citing various types of personnel-associated deviations is that staff are better trained on paper than through hands-on practical experience. However, the focus on training has been more on compliance with regulations and less on the efficacy and success of transferring practical skills. Closing these skill gaps and resolving the associated drawbacks necessitate a thorough review and rethinking of current training practices established in the biopharmaceutical industry.


The Regulatory Perspective
From a regulatory point of view, Part 211 Current Good Manufacturing Practice for Finished Pharmaceuticals, Section 211.25 on personnel qualification, states that each person engaged in the manufacture, processing, packing, or holding of a drug product shall have education, training, and experience (or any combination thereof) to enable that person to perform the assigned functions. The same is required in EudraLex Volume 4 Chapter 2 on Personnel.
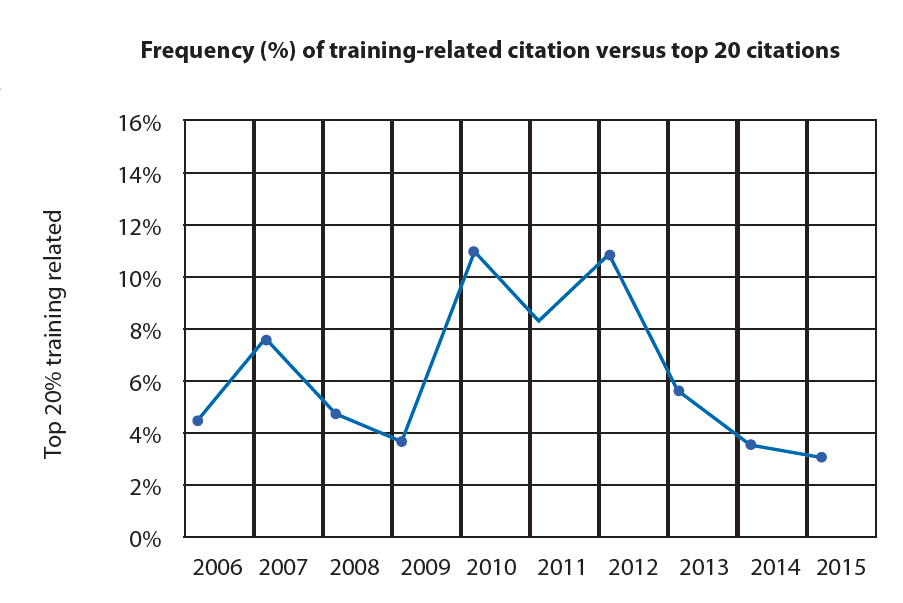
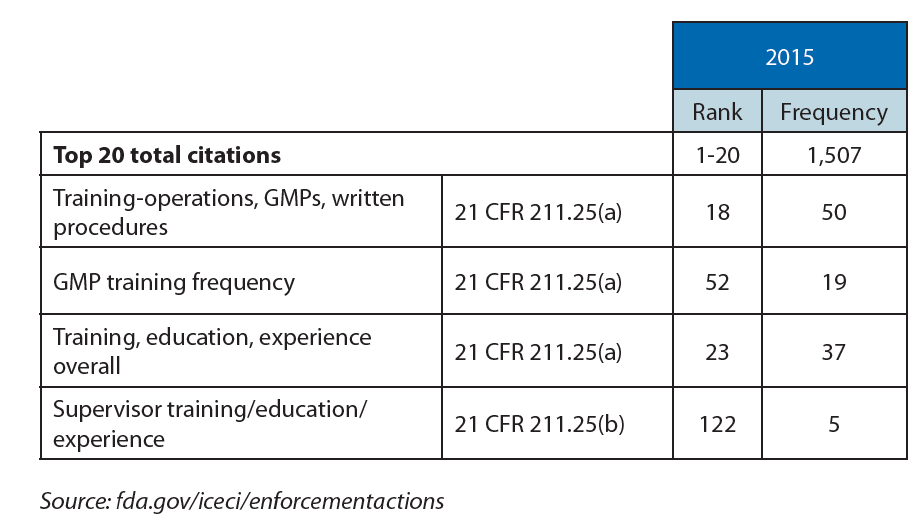
Analysis of the Form 483 citations published by the FDA over the last 10 years reveals recurrent issues of compliance with documented procedures, absence of documented procedures, and/or adequate training. These findings represent 25-35% of FDA citations, even though Section 211.100 states that written procedures for production and process control are required and need to be followed and completed at the time of execution.
The combination of missing or insufficient procedures and ineffective training leads to deviations and lost batches, all of which requires significant effort in terms of investigation and implementation of corrective and preventative action (CAPA).
The current situation is a major challenge for the biopharma industry, given that its origins need to be investigated and the absence of a trend towards a reduction explained. Findings often identify that written procedures cannot be followed by operators because they are either inadequate or not understandable; on top of this, if training systems are not efficacious, operators may execute procedures without the framework that enables continuous success and compliance.
Furthermore, investigations into root causes of operational failures are frequently unsuccessful. Root causes tend to be assigned to ‘operator error’ without any real evidence that errors were made, and ‘retraining’ is arbitrarily assigned as CAPA. However, regulatory agencies are increasingly taking the position that if it is not known what error was committed, then retraining alone is an unsatisfactory resolution. Regulators are also increasingly citing the number of unresolved investigations as evidence that the standards of the CAPA processes and training are inadequate and, therefore, by extension, the manufacturer’s GMP standards are probably unacceptable, which can have far-reaching regulatory implications.
Industry Training
To fulfill Janet Woodcock’s vision (Director at CDER-FDA) of a pharma industry working at a high-quality level without needing major regulatory oversight, the biopharma industry is required to initiate a multi-pronged approach to:
- Create straightforward, clear, and crisp written documentation
- Create efficient training environments and GMPs
- Create an ecosystem that encourages operator judgment and proactivity
- Create an ecosystem that enables proper root-cause investigation to support active risk reduction by executing meaningful CAPAs
- Create the basis for a more entwined business relationship between suppliers and end users to support strong collaboration
(particularly since suppliers deliver complete solutions today)
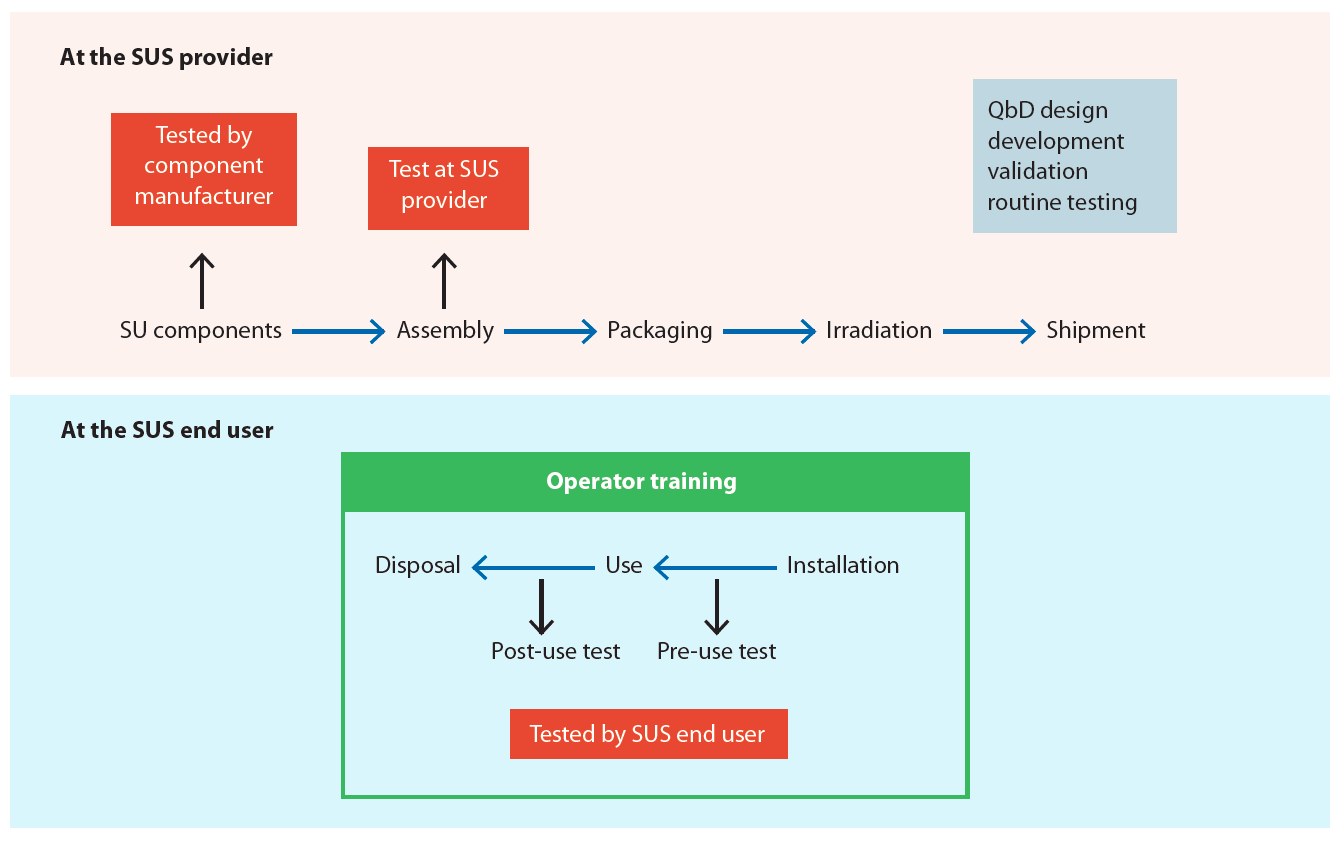
It will be the successful combination of the different prongs that will drive evolution of the current situation towards operational excellence. Especially in the incorporation of single-use technologies (SUT) training; this is seen as a key contributor to the reduction of operator error in such settings, alongside with good design principles of single-use components and systems (see Figure 3).
The fundamental principle when designing a product or service is to understand the demands and needs of the end user. User-centred design (UCD) is a development method that places the end user at the centre of the design process and ensures products will be easy to use. The International Usability Standard, ISO 13407, specifies the principles and activities of the UCD process:
- The design is based upon an explicit understanding of users, tasks, and environments
- Users are involved throughout design and development
- The design is driven and refined by user-centred evaluation
- The process is iterative
- The design addresses the whole user experience
- The design team includes multidisciplinary skills and perspectives
As products and operations become more complex, the use of robust design principles becomes even more important to reduce or even eliminate operator errors associated with efficient training methods.
Training is Critical
The implementation of SUT illustrates why ‘old school’ training methods cannot meet the new biopharma industry requirements. Successful return on investment of SUT is secured by honing the necessary new skillsets that operators must have to ensure proper process operations. Moreover, the past decades of SUT improvements include the recent approach by suppliers to design single-use systems considering their usability. These combined efforts are making a solid contribution to the elimination of traps detrimental to productivity, with fewer deviations qualified as human errors. Ultimately, training for competencies fosters compliance to the GMP process and patient safety.
The development of SUT has significantly changed the interaction and collaboration between end users and suppliers. Both parties need to agree on training packages delivered and embedded together with ready-to-use, end-to-end manufacturing process solutions. Supplier expertise can be instrumental in building appropriate training packages and writing working instructions. This has been acknowledged by some suppliers who have started to develop production personnel training initiatives.
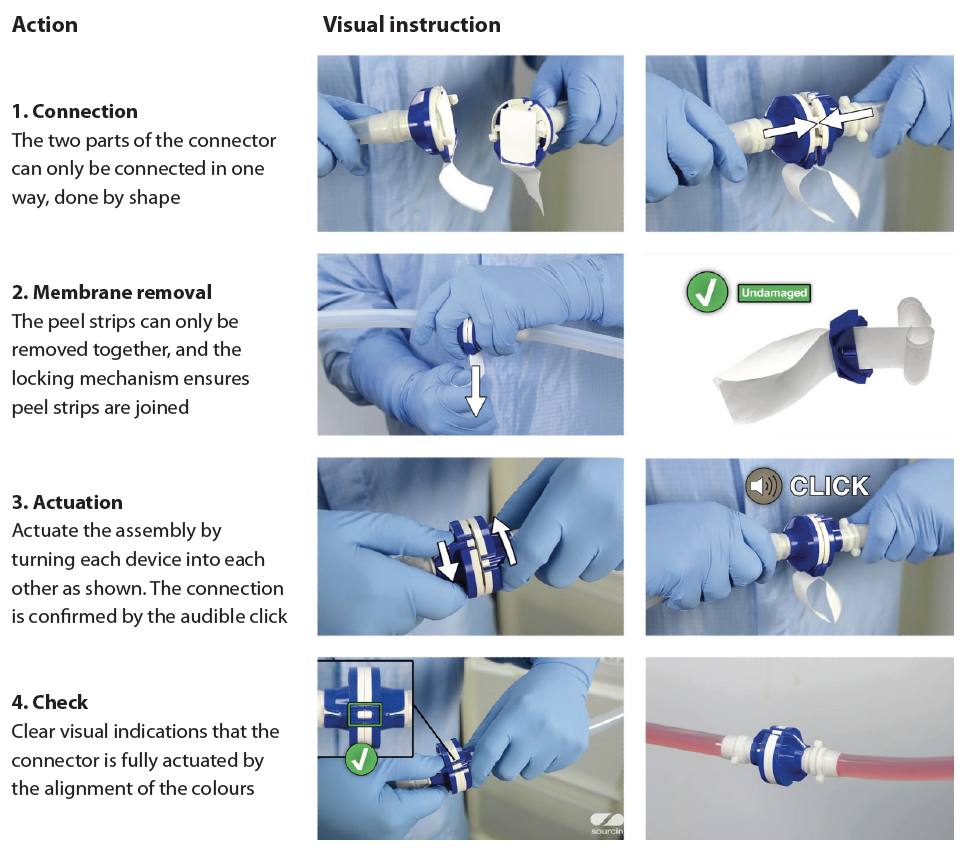
For instance, aseptic processing is a highly critical application in the biopharma industry as any breach in sterility can have an enormous impact on patient safety. Sterile connectors are now widely used in biopharmaceutical processing to allow the connecting of two sterile entities even in uncontrolled environments (see Figure 4, page 44).
Additionally, efficient personnel training strategies should be developed considering the human learning aptitude to ensure efficacious transfer of skills to personnel beyond regulatory documentation requirements. While good industry design principles can result in more effective and robust use of SUT with a concomitant compliance increase in the biopharma industry, they do not eliminate the requirement for better training. However, when used in association with improved and more robust training methods, they will significantly increase the robustness of the use of these newer technologies in the manufacturing environment.
Challenges and Solutions for Training
Like any manufacturing sector, training requirements in a typical biotech plant environment are realised in a combination of live demonstration, written instruction, and supervision. The focus is GMP compliance and patient safety. Many biotech training systems are still based on ‘read and understand’ rather than ‘show’ methods. Written training instructions and operator demonstrations remain the pillars of training in most plants, while topics requiring manual and spatial perception skills are only lightly considered even though it is well understood that focusing on written instruction at the expense of visual and oral instruction will deliver inferior training outcomes.
Furthermore, training is frequently given to a few highly skilled operators, which can result in an immediate resource issue when an experienced operator is absent from the shop floor. Successful training is unit task dependent and involves repetitions to minimise operator-to-operator variability, thus requiring extensive time.
Training needs to support introducing new technologies, consistent operations at all sites, and the expansion of capacity. Implementing SUT in drug commercial production is a good example of how new technologies can change requirements for skillsets and expertise. Fulfilling these requirements poses a major challenge when recruiting new personnel at multiple sites, including emerging countries with language and cultural barriers. Therefore, creating right-first-time training content is a major success factor driving the need for a new set of training tools and methodologies that are expandable and adaptable to stakeholders.
The application of training should be user focused so that the end-user can easily recall instructions. As discussed previously, there is no escape from watching how to perform a given task. Therefore, more visual training material is needed combined with a shorter distance between the instructor knowledge and the trainees. Access to learning materials must be simple and ideally self-directed to give individuals the ability to decide their best teachable time.
Mediabooks are a new, holistic training methodology that takes the form of visual libraries of instructional videos combining images, animations, and live-captured videos that include expert and quality assurance teams. They are accessible on personal computers and can be securely integrated at the point of operation into portable smart media tools such as laptops, tablets, and iPads.
Mediabooks have the capability to document, demonstrate, and explain complex manual steps unambiguously and consistently. Content can also visualise or demonstrate what is happening behind the ‘walls’ by providing the principles applied and the rationale of key process attributes to enable operators to gain a better understanding.
Based on microlearning (learning in minutes), which does not overload the trainee agenda, mediabooks provide access to instructors demonstrating expertise or skillsets for a given task at any time and as many times as is necessary for the trainee to get attuned to the process. Using these tools can:
- Contribute to GMP compliance by reconciling tacit and explicit knowledge and previewing the instructions by a wide range of stakeholders, such as quality assurance, regulatory, and process sciences, which ensures critical techniques are correct and uniform
- Provide off-line training capabilities that reduce the expense and required time for training, allow for multiple repetitions to be performed, reduce the risk to operations, products, patients, and the drain on the time and expertise of skilled operators with the result that they can be released for their primary role of running the operation
- Speed up the training-to-knowledge process by assisting workers in non-frequent but critical activities prior to being performed (e.g., system installation or column packing) and reducing the risks of losing knowledge and expertise as skilled operators move to other roles
- Contribute to operational excellence by increasing the chances of success
when implementing process changes and at tech transfer and contributing to eliminating deviations in production
Building Success into Training
The goal of training in the biopharma industry is to gain efficacy. This can be achieved by blending face-to-face, hands-on, and interactive eLearning (self-inducted) training methods. This combination provides many opportunities
to monitor competencies and to mentor trainees. The data generated also guide the trainers in adapting training curricula for each audience. Additionally, compelling short sessions of digital training complement class or workshop sessions without eating up the time and resources needed, and video tutorials and on-line assessments help trainers deliver knowledge without altering their time of presence.
From a company perspective, using a secured private cloud ensures that instructions are conveyed at the point of use. It also provides an economy of scale since gated access, permissions, and rights are assigned to user profiles allowing the sharing of expert knowledge at will. Rapid deployment does not require additional capital expenditure and provides a host of benefits, including:
- Full visibility of the knowledge assets
- Control over the quality of the learning assets
- On-demand training in the trainee’s hands
- Publishing on multiple platforms with learning analytics
- Improved integration of training into the company’s quality and learning management systems
To build successful training platforms, it is important to stay ahead of trends and work with experts in the space. By watching the mediabooks (via secured platforms), companies are able to support the overall learning process, and, with a scalable tool that provides continuity of training content, manufacturing sites gain the ability to provide relevant, consistent, and modern teaching methods for overall process success.

